Research on the molecular mechanism involved in HCC immunotherapy failure in HEPATOLOGY
2023.02.28Recently, SULT2B1 CS DOCK2 Axis Regulates Effector T Cell Exhaustion in Hepatocellular Carcinoma Microenvironment was published in the journal HEPATOLOGY by Professor Xu Xiao (corresponding author) from Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine.

Hepatocellular carcinoma (HCC), the most common primary liver tumor, is one of the major causes of cancer-related deaths worldwide and is mainly associated with hepatitis B or C virus infection, alcohol abuse, and metabolic syndrome. The preferred treatment methods for HCC vary from region to region. When tumors develop inside the liver, locoregional treatments, including resection, percutaneous ablation, transarterial chemoembolization, and liver transplantation, are recommended based on tumor location, tumor burden, and comorbidities. For advanced HCC, oral multiple tyrosine kinase inhibitors (TKIs) have been used as the first- or second-line therapy in the clinic. However, their therapeutic effects are not very satisfactory owing to the limited efficacy in prolonging the survival of patients. In order to explore the involved molecular mechanism, this study using machine learning and multiplex immunohistochemistry analysis combined other biological experiments to find: 1) DOCK2 controls CD8+ T cell infiltration in HCC; 2) CS synthesized by SULT2B1 in tumor cells promotes effector T cell exhaustion; 3) the usage of conventional drugs affects immunotherapy efficacy in HCC patients.
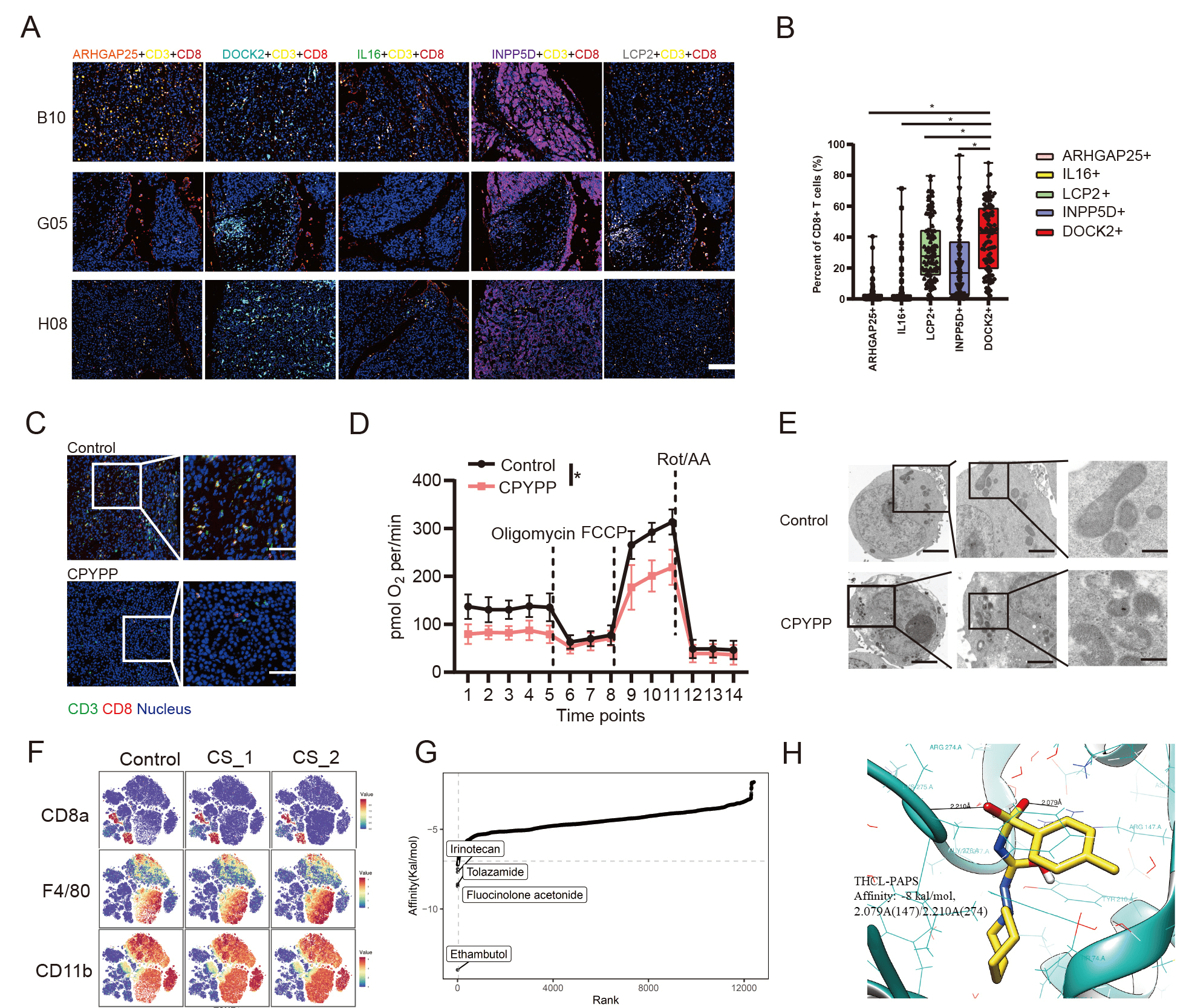
Fig.1 Overview of SULT2B1-CS-DOCK2 axis regulating CD8+ T exhaustion in HCC TME. A) TSA analysis about indicated protein positive CD8+T in HCC tissue; B) Statistic analysis of A. C) CPYPP suppresses DOCK2+ CD8T infiltration in HCC. D) Seahorse analysis of CD8+T treated with CPYPP. E) Electron microscope picture of mitochondrion. F) CyTOF analysis of tumor-infiltrated cells. G) Virtual screen of small molecules binding to SULT2B1. H) Structure docking of THCL and PAPS.
In this paper, our group using RNA sequencing, flow cytometry analysis, and mouse HCC models to demonstrate that DOCK2 inactivation accounted for infiltrated CD8+ T cell exhaustion in tumors. Using quasi-targeted metabolomics, mass spectrum, and mass cytometry by time of flight analysis, we found that cholesterol sulfate (CS) synthesized by sulfotransferase 2B1 (SULT2B1) in tumor cells suppressed DOCK2 enzymatic activity of T cells. Through virtual screening, molecular docking simulation, and experiments validation, we demonstrated that tolazamide reversed DOCK2 inactivation-mediated CD8+ T cell exhaustion and enhanced anti-PDL1 antibody + Apatinib immunotherapeutic effects on HCC. This study firstly indicates that DOCK2 controls CD8+ T cell infiltration in HCC, and CS synthesized by SULT2B1 in tumor cells promotes effector T cell exhaustion. The findings suggest that the usage of conventional drugs affects immunotherapy efficacy in HCC patients.
Wang Shuai, Wang Rui and Xu Nan are co-first authors. Xu Xiao and Lu Di are co-corresponding authors.


